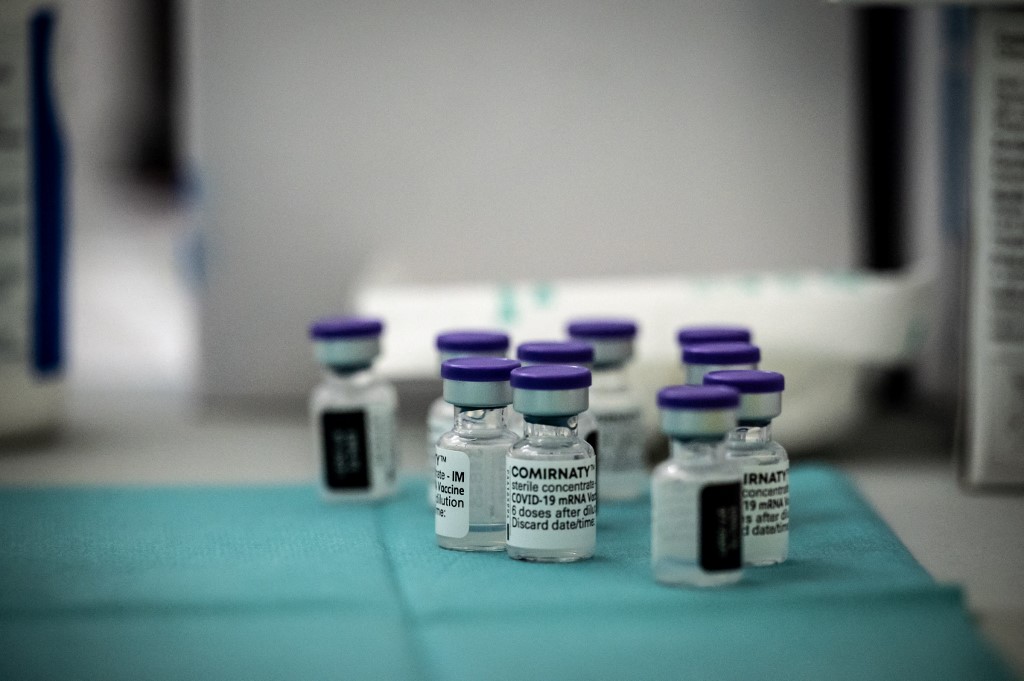
EMA recommends first COVID-19 vaccine for authorisation in the EU News 21122020 Comirnaty is now authorised across the EU. AstraZeneca submitted date for vaccine approval says UKs Hancock.
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
The WHO prequalification process will assess additional clinical data generated from vaccine trials and deployment on a rolling basis to ensure the vaccine meets the necessary standards of quality safety and efficacy for broader availability.

Vaccine approval date. It has also been approved in Canada. At the end of January it was announced the Janssen vaccine was effective at preventing COVID-19 in trials and now the jab is awaiting approval from. 8 January 2021 see all updates.
The COVID-19 vaccine developed by Moderna has today been given regulatory approval for supply by the Medicines and Healthcare products Regulatory Agency. If the FDA grants full approval Pfizer would be able. WHO also listed the PfizerBioNTech vaccine for emergency use on 31 December 2020.
Food and Drug Administration issued the first. Post-approval follow -up for safety and efficacy. This follows the granting of a conditional marketing authorisation by the European Commission on 21 December 2020.
This will be prioritized according to the populations identified by the CDCs Advisory Committee on Immunization Practices ACIP guidelines. The World Health Organization has approved the PfizerBioNTech vaccine for emergency use paving the way for lower and middle-income countries to. In principle conditional marketing authorisation for a COVID-19 vaccine could be based on review of at least 6 weeks post -vaccination safety data.
The Company plans to deliver 100 million single-shot vaccines to the US. Vaccines typically require years of research and testing before reaching the clinic but in 2020 scientists embarked on a race to produce safe and effective coronavirus vaccines in record time. Pfizer reported that it would continue.
Government will manage allocation and distribution of the vaccine in the US. December 14 2020 -- The CDC signed off on the Pfizer-BioNTech COVID-19 vaccine the agency announced on Sunday afternoon completing the final approval for. No matter what only a safe effective vaccine will get our approval.
Full FDA approval however requires a so-called Biologics License Application or BLA. Pfizer said it plans to seek full approval of its vaccine from the FDA in April 2021. Regulators in December 2020 here.
After clearing final Phase III clinical trials the Pfizer-BioNTech vaccine was granted approval for emergency use by US. COVID-19 and the FDA. 30 rows On December 11 2020 the US.
Reactions to vaccines occur within 4 -6 weeks from vaccination. By then it will have collected 6 months of safety data. Pfizer and BioNTech are planning to submit a Biologics License Application BLA during the first half of.
85 rows FDA commissioner. Dr Hahn discusses the. During the first half of 2021.
Hunt reiterated his governments advice that the timeline for a decision on approval is expected by the end of January with the first vaccines planned to be delivered in March. The vaccine has already received approval in the UK and health officials began vaccinating people there on Dec. Im delighted to be able to tell you that the Oxford AstraZeneca vaccine developed here in the UK has submitted its full.